In the biopharmaceutical industry, an astonishing 70% of drug development failures are attributed to issues related to transport and supply chain management. The introduction of advanced systems like the antibody Registration system (ARS) is crucial for addressing these challenges.
Understanding the Antibody Registration System and Its Transport Attributes
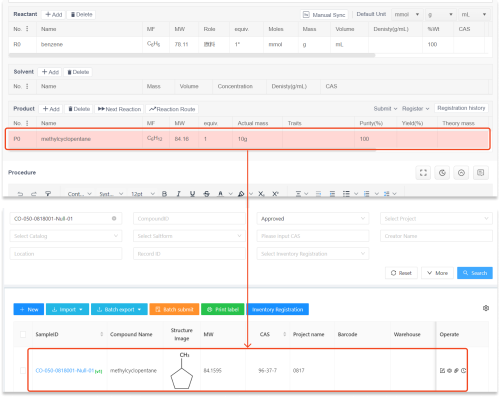
The Antibody Registration System serves as a pivotal tool in managing the lifecycle of antibodies from discovery through distribution. One of its key features is enhancing transport attributes by ensuring that each antibody’s stability, integrity, and efficacy are maintained throughout transit. This system provides real-time data on temperature control, humidity levels, and other critical factors affecting product quality during transportation. Moreover, it significantly contributes to Supply Chain Visibility by allowing stakeholders to track shipments at every stage with precision.
Diving Deeper into Compound Registration Systems and Supply Chain Visibility
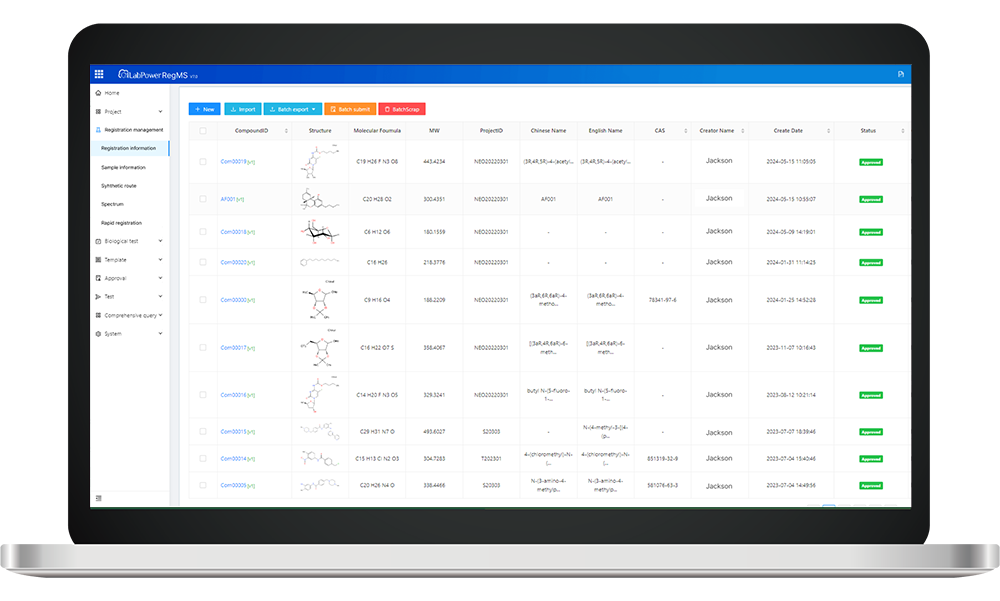
compound registration systems play a vital role in bolstering Supply Chain Visibility within biopharmaceutical logistics. These systems facilitate comprehensive tracking of compounds from their initial synthesis through various stages until they reach end-users. By integrating detailed documentation regarding each compound’s properties—such as batch numbers, expiration dates, and storage conditions—these systems enhance transparency across the supply chain. As a result, stakeholders can make informed decisions quickly while minimizing risks associated with product recalls or losses due to improper handling.
Key Features of Neotrident in Enhancing Supply Chain Visibility
- Real-Time Monitoring: Neotrident offers continuous monitoring capabilities that provide live updates on shipment conditions such as temperature fluctuations or delays.
- User-Friendly Interface: The platform boasts an intuitive interface that allows users to easily access critical information about their products’ journey through the supply chain.
- Error Reduction: Automated alerts help reduce human errors by notifying relevant parties immediately when predefined thresholds are breached during transport.
- Sustainability Tracking: Neotrident incorporates sustainability metrics into its visibility framework, enabling companies to assess environmental impacts throughout their logistics processes.
- Crisis Management Tools: In case of disruptions or emergencies within the supply chain network, Neotrident equips users with tools for rapid response planning and execution.
A Concluding Perspective on Antibody Registration Systems’ Impact on Supply Chain Visibility
The integration of Antibody Registration Systems marks a significant advancement in improving Supply Chain Visibility within biopharmaceuticals. By providing essential insights into transport attributes such as stability monitoring and real-time tracking capabilities offered by platforms like Neotrident, these systems not only safeguard product integrity but also foster greater accountability among all stakeholders involved in drug delivery processes. Ultimately, this leads us toward more efficient operations capable of meeting both regulatory demands and patient needs effectively.

